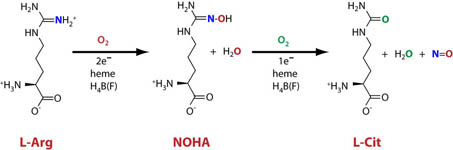
Nitric oxide synthase (NOS) proteins are heme-based monooxygenase enzymes that convert L-arginine to L-citrulline and nitric oxide (NO). NO is a key diffusible signaling molecule and cytotoxic agent in mammals. As a result, its production in cells is a target for drug design in many different capacities.
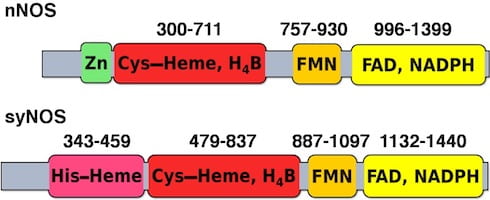
Several cyanobacteria have been identified that possess a NOS composed of NOSox-NOSred. Surprisingly there is also an additional globin domain (NOSg) unprecedented in any other NOS. We have isolated and purified a NOS enzyme from Synechococcus sp. PCC 7335 (syNOS) and confirmed that it produces NO from arginine, however, we have discovered that NOSg rapidly oxidizes all NO to nitrate. This appears to be a futile process, but NOSg may serve as a regulatory mechanism to “turn off” NO production. We are investigating factors controlling electron transfer to NOSg and NOSox using spectroscopy, ESR, X-ray crystallography, and other biochemical and structural methods.
A nitric oxide synthase-like protein from Synechococcus produces NO/NO3 – from l-arginine and NADPH in a tetrahydrobiopterin- and Ca2+-dependent manner.
Nitric oxide synthases (NOSs) are heme-based monooxygenases that convert l-Arg to l-citrulline and nitric oxide (NO), a key signaling molecule and cytotoxic agent in mammals. Bacteria also contain NOS proteins, but the role of NO production within these organisms, where understood, differs considerably from that of mammals. For example, a NOS protein in the marine cyanobacterium Synechococcus sp. PCC 7335 (syNOS) has recently been proposed to function in nitrogen assimilation from l-Arg. syNOS retains the oxygenase (NOSox) and reductase (NOSred) domains present in mammalian NOS enzymes (mNOSs), but also contains an N-terminal globin domain (NOSg) homologous to bacterial flavohemoglobin proteins. Herein, we show that syNOS functions as a dimer and produces NO from l-Arg and NADPH in a tetrahydrobiopterin (H4B)-dependent manner at levels similar to those produced by other NOSs but does not require Ca2+-calmodulin, which regulates NOSred-mediated NOSox reduction in mNOSs. Unlike other bacterial NOSs, syNOS cannot function with tetrahydrofolate and requires high Ca2+ levels (>200 μm) for its activation. NOSg converts NO to NO3 – in the presence of O2 and NADPH; however, NOSg did not protect Escherichia coli strains against nitrosative stress, even in a mutant devoid of NO-protective flavohemoglobin. We also found that syNOS does not have NOS activity in E. coli (which lacks H4B) and that the recombinant protein does not confer growth advantages on l-Arg as a nitrogen source. Our findings indicate that syNOS has both NOS and NO oxygenase activities, requires H4B, and may play a role in Ca2+-mediated signaling.
Picciano AL, Crane BR. A nitric oxide synthase-like protein from Synechococcus produces NO/NO3— from l-arginine and NAPDH in a tetrahydrobiopterin- and Ca2+-dependent manner. J Biol Chem. 2019 Jul 5;294(27):10708-10719. PubMed PMID: 31113865.