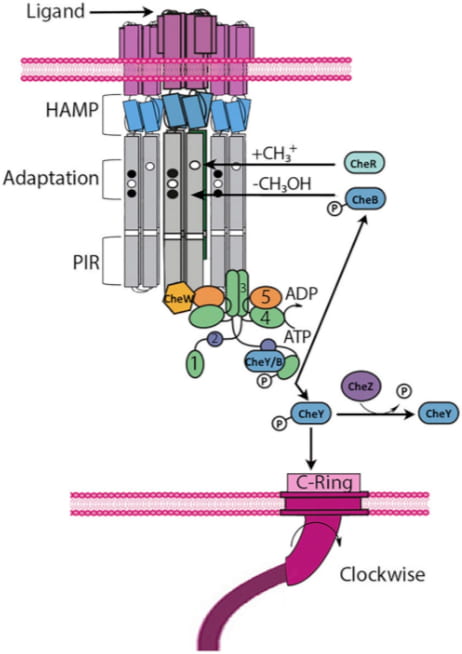
Bacterial chemotaxis is the motile response of prokaryotes to external stimuli and has long stood as a model system for understanding transmembrane signal transduction, intracellular information propagation, and motility. Many human pathogens, including Vibrio cholerae, Helicobacter pylori, Treponema pallidum (syphilis), and Borrelia burgderfori (lyme disease) rely on chemotaxis for infectivity. The signaling system underlying chemotaxis exhibits amazing sensitivity, robustness, dynamic range, and even a rudimentary molecular memory. Bacteria are capable of sensing changes in chemical concentrations of less than one percent over background concentrations ranging five orders of magnitude.
The components intrinsic to this cooperative excitation response and feedback control are well characterized in the model system E. coli, yet the biochemical mechanisms responsible for function remain unclear. Indeed, the E. coli cytoplasmic membrane comprises of an extensive multi-component transmembrane assembly of receptors (MCPs) along with histidine kinases (CheA) and coupling proteins (CheW). Ultimately, we aim to understand how cell surface receptors interact with and modulate the activity of the central chemotaxis histidine kinase CheA.
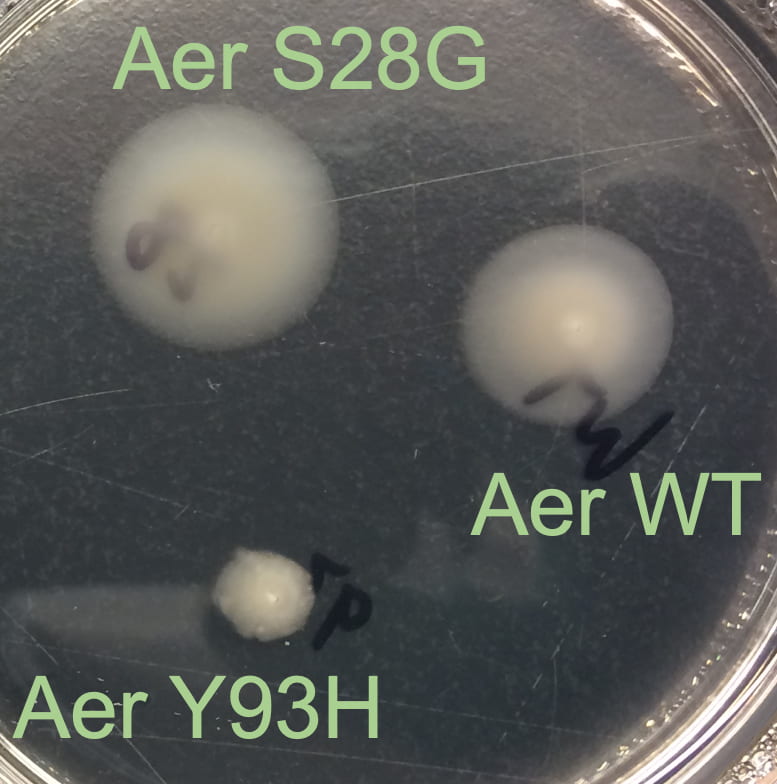
Aerotaxis protein:
Chemical sensing is mediated by homodimer transmembrane chemoreceptors, including the E. coli energy sensor for motility, Aer. Energy taxis, a variant of chemotaxis universal in motile aerobic bacteria, describes the motile response to respiratory changes, including those induced by oxygen and other electron receptors, such as nitrate and fumarate. Energy taxis is required to stimulate movement away from regions of high nutrient concentration, but low oxygen concentration.
Structure and chemistry of lysinoalanine crosslinking in the spirochaete flagella hook.
The flagellar hook protein FlgE from spirochaete bacteria self-catalyzes the formation of an unusual inter-subunit lysinoalanine (Lal) crosslink that is critical for cell motility. Unlike other known examples of Lal biosynthesis, conserved cysteine and lysine residues in FlgE spontaneously react to form Lal without the involvement of additional enzymes. Oligomerization of FlgE via its D0 and Dc domains drives assembly of the crosslinking site at the D1-D2 domain interface. Structures of the FlgED2 domain, dehydroalanine (DHA) intermediate and Lal crosslinked FlgE subunits reveal successive snapshots of the reaction. Cys178 flips from a buried configuration to release hydrogen sulfide (H2S/HS–) and produce DHA. Interface residues provide hydrogen bonds to anchor the active site, facilitate β-elimination of Cys178 and polarize the peptide backbone to activate DHA for reaction with Lys165. Cysteine-reactive molecules accelerate DHA formation, whereas nucleophiles can intercept the DHA intermediate, thereby indicating a potential for Lal crosslink inhibitors to combat spirochaetal diseases.
Lynch MJ, Miller M, James M, Zhang S, Zhang K, Li C, Charon NW, Crane BR. Structure and chemistry of lysinoalanine crosslinking in the spirochaete flagella hook. Nat Chem Biol. 2019 Oct;15(10):959-965. PMID: 31406373
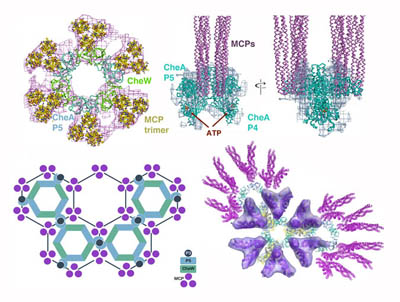
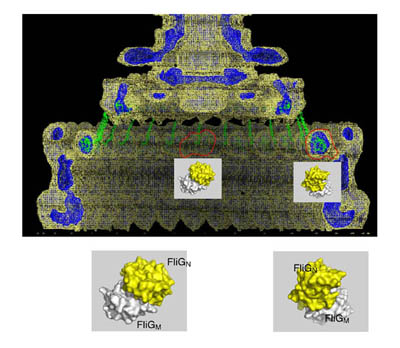
Regulation of the chemotaxis histidine kinase CheA: A structural perspective.
Bacteria sense and respond to their environment through a highly conserved assembly of transmembrane chemoreceptors (MCPs), the histidine kinase CheA, and the coupling protein CheW, hereafter termed “the chemosensory array”. In recent years, great strides have been made in understanding the architecture of the chemosensory array and how this assembly engenders sensitive and cooperative responses. Nonetheless, a central outstanding question surrounds how receptors modulate the activity of the CheA kinase, the enzymatic output of the sensory system. With a focus on recent advances, we summarize the current understanding of array structure and function to comment on the molecular mechanism by which CheA, receptors and CheW generate the high sensitivity, gain and dynamic range emblematic of bacterial chemotaxis. The complexity of the chemosensory arrays has motivated investigation with many different approaches. In particular, structural methods, genetics, cellular activity assays, nanodisc technology and cryo-electron tomography have provided advances that bridge length scales and connect molecular mechanism to cellular function. Given the high degree of component integration in the chemosensory arrays, we ultimately aim to understand how such networked molecular interactions generate a whole that is truly greater than the sum of its parts. This article is part of a Special Issue entitled: Molecular biophysics of membranes and membrane proteins.
Muok AR, Briegel A, Crane BR. Regulation of the chemotaxis histidine kinase CheA: A structural perspective. Biochim Biophys Acta Biomembr. 2019 Jul 30:183030. PMID: 31374212